Direct identification of ALK and ROS1 fusions in non-small cell lung cancer from hematoxylin and eosin-stained slides using deep learning algorithms | Modern Pathology
Automated Workflow for Preparation of cDNA for Cap Analysis of Gene Expression on a Single Molecule Sequencer | PLOS ONE
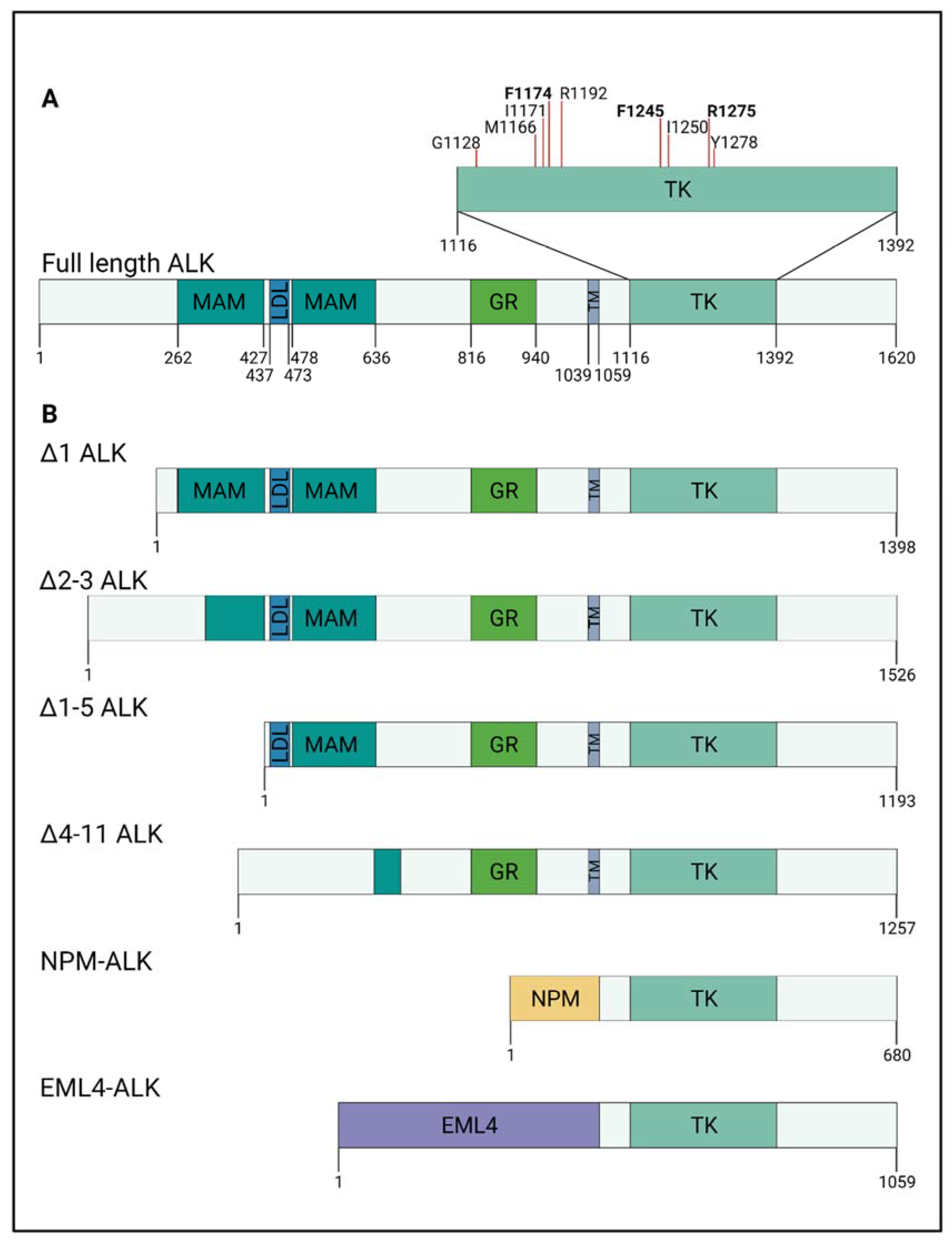
IJMS | Free Full-Text | Defining Pathological Activities of ALK in Neuroblastoma, a Neural Crest-Derived Cancer

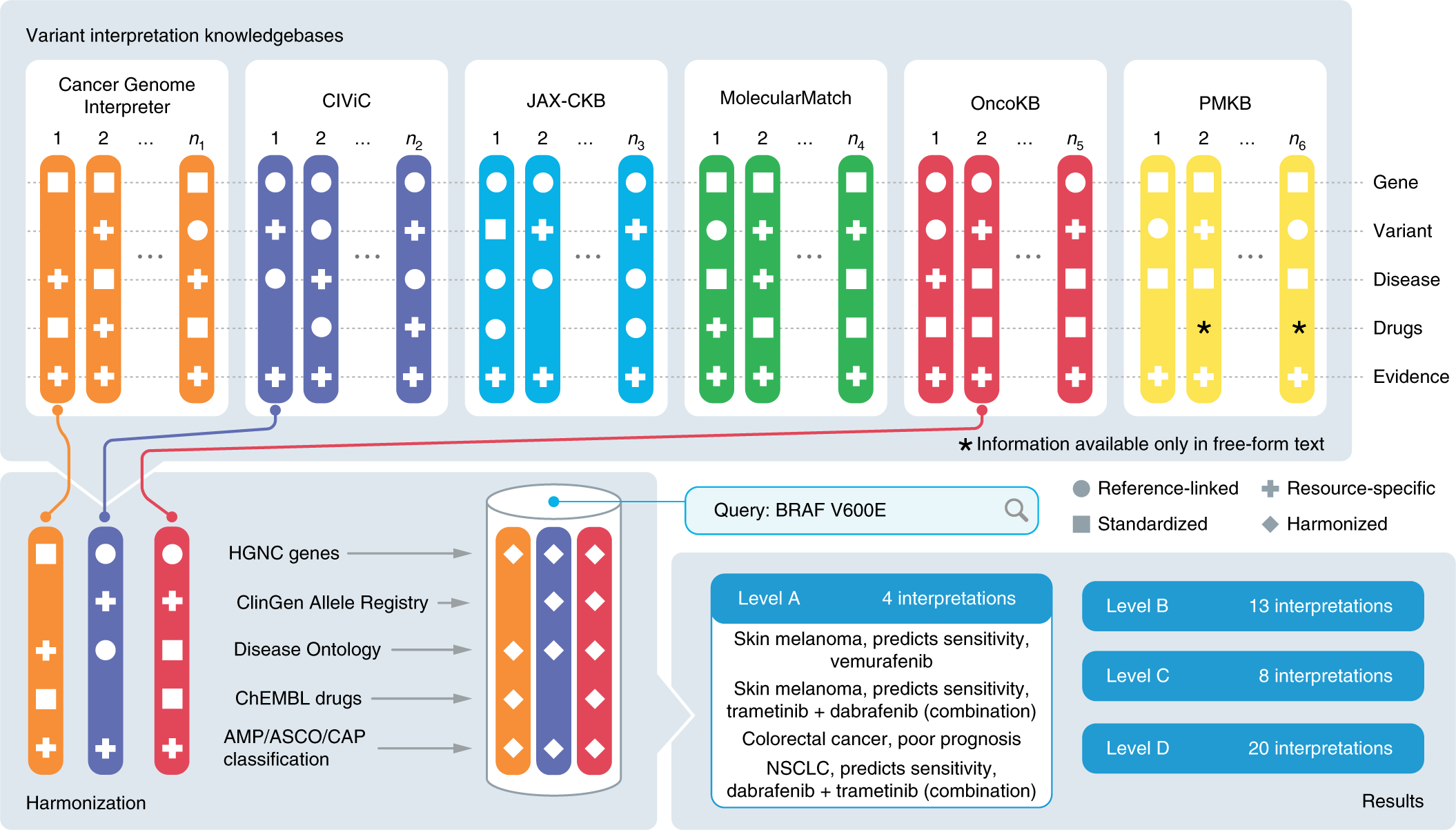
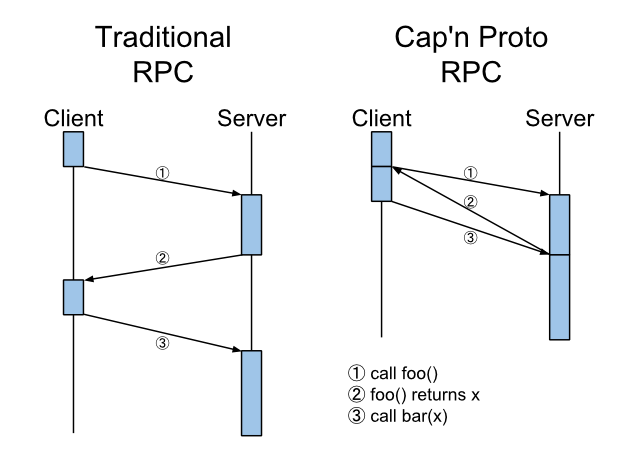
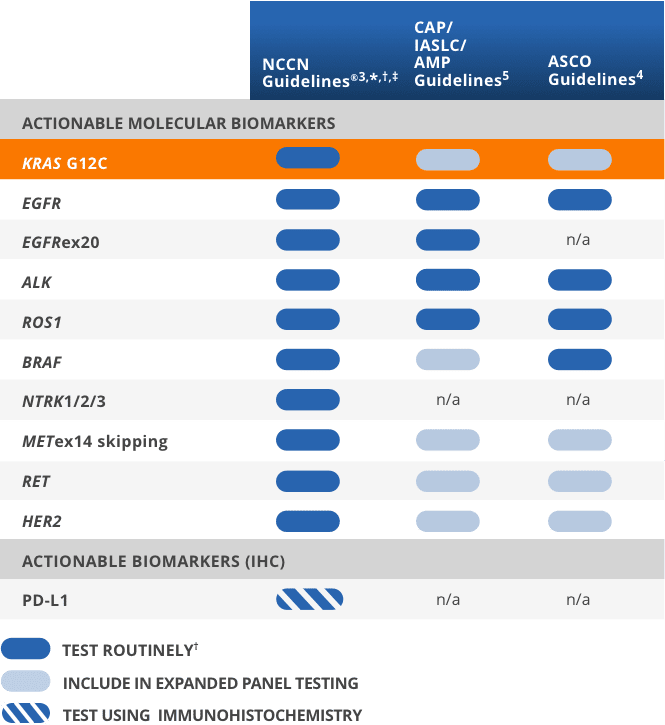
Expert opinion on NSCLC small specimen biomarker testing — Part 2: Analysis, reporting, and quality assessment | SpringerLink
Template for Reporting Results of Biomarker Testing of Specimens From Patients With Non-Small Cell Carcinoma of the Lung

Rationale and Design of the Targeted Agent and Profiling Utilization Registry Study | JCO Precision Oncology

Camrelizumab plus apatinib as second-line treatment for advanced oesophageal squamous cell carcinoma (CAP 02): a single-arm, open-label, phase 2 trial - The Lancet Gastroenterology & Hepatology

PDF) Cap 1 Messenger RNA Synthesis with Co-transcriptional CleanCap® Analog by In Vitro Transcription
Protocol for the Examination of Resection Specimens From Patients With Primary Non-Small Cell Carcinoma, Small Cell Carcinoma, o
J2G-GH-JZJK Protocol(b) A Phase 2 Study of Oral Selpercatinib (LOXO-292) in Patients with Advanced Solid Tumors, Including RET F